[Shyi-Dong Yeh] A conserved helix in C-terminal region of watermelon silver mottle virus NSs protein is imperative for protein stability affecting self-interaction, RNA silencing suppression and pathogenicity
Huang, C. H., Foo, M. H., Raja, J. A. J., Tan, Y. R., Lin, T. T., Lin, S. S., and Yeh, S. D., 2020 Molecular Plant-Microbe Interactions
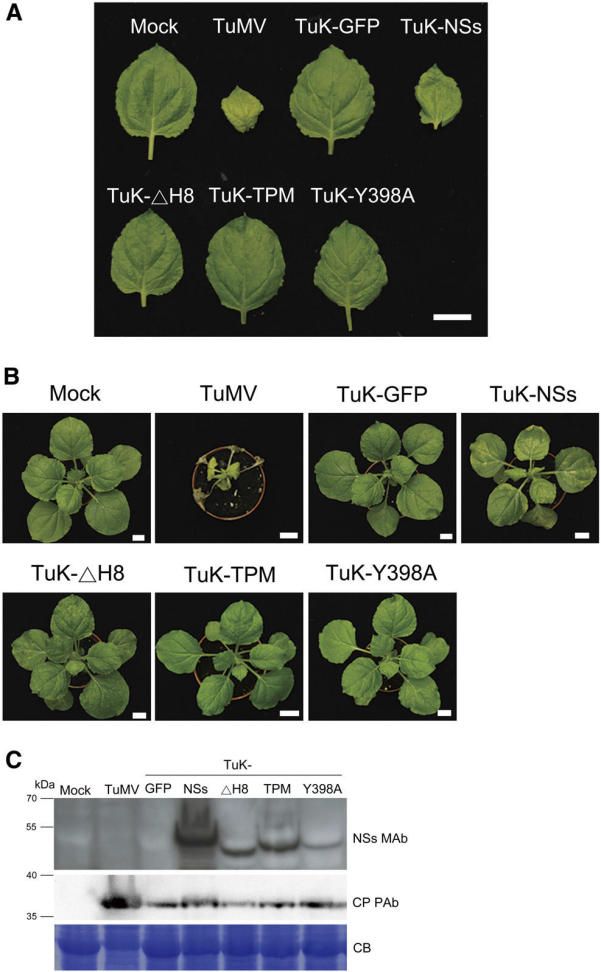
In orthotospovirus, the nonstructural protein S (NSs) is the RNA-silencing suppressor (RSS) and pathogenicity determinant. Here, we demonstrate that a putative a-helix, designated H8, spanning amino acids 338 to 369 of the C-terminal region of the NSs protein, is crucial for stabilizing NSs protein through self-interaction to maintain normal functions of RSS and pathogenicity, but not for NSs siRNA binding activity.
Article source: https://doi.org/10.1094/MPMI-10-19-0279-R
